Misrepresentation and distortion of research in biomedical literature.
Publication in peer-reviewed journals is an important step in the scientific course of. However, publication will not be merely the reporting of info arising from an easy evaluation thereof.
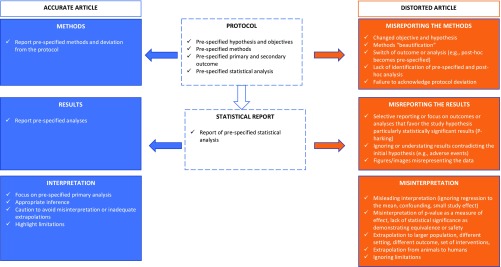
Authors have broad latitude when writing their reviews and could also be tempted to consciously or unconsciously “spin” their examine findings.
Spin has been outlined as a particular intentional or unintentional reporting that fails to faithfully replicate the character and vary of findings and that might have an effect on the impression the outcomes produce in readers.
This article, based mostly on a literature evaluate, reviews the assorted practices of spin from misreporting by “beautification” of strategies to misreporting by misinterpreting the outcomes. It supplies knowledge on the prevalence of some kinds of spin in particular fields and the potential results of some varieties of spin on readers’ interpretation and research dissemination.
We additionally focus on why researchers would spin their reviews and potential methods to keep away from it.
Can there be an ethical obligation to take part in biomedical research?
In medical drugs, the ethical obligation to look after the person affected person is absolute. Patient care means a minimum of and by adverse phrases to reduce any danger of therapy. In this context, the query arises concerning the compatibility of medical ethics and human biomedical research ethics.
Or conversely, is there a typical floor between the 2? At the other finish of the sphere between medical ethics and biomedical research ethics is the proposal of an obligation to take part in biomedical research, which is argued for on the idea of biomedical data being a public good accessible to the group as a complete.
While affected person accrual throughout a medical investigation would definitely be facilitated by compulsory research participation, and the information obtained would be-at first sight-more consultant for the inhabitants studied, the nonetheless possible refusal to take part could be stigmatizing and as such detrimental for the patient-physician relation.
This essay seeks to offer a reply to the titled query by specializing in points comparable to particular person vs frequent medical claims, shared grounds between the 2 and an necessary doc of medical research ethics, that’s the Nuremberg code.