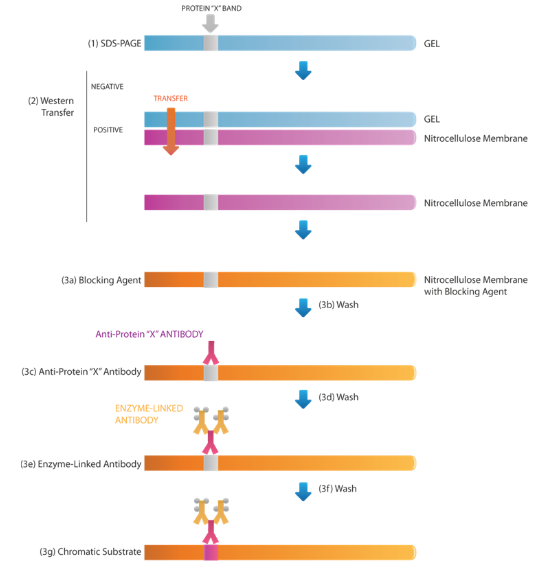
Western Blot Protocols (part 3) – Antibody Incubation & Gel Visualization
1. Blocking
Visualization of proteins in membranes (non-compulsory):
As an non-compulsory step, we will confirm the protein had been transferred efficiently by staining the membrane with ponceau purple. Incubate the membrane in ponceau for five minutes and wash with water till the bands are clear. After verification, the bands can then be destained by persevering with to scrub with water or TBS-tween till the dye is totally eliminated. When utilizing a PVDF membrane, re-activate the membrane with methanol then wash once more in TBS-Tween. (Figure 1 1a, 1b, 1c)
As non-particular binding of antibodies to the membrane is detrimental to the specificity and sensitivity of the assay, it’s important to “block” areas not already occupied by proteins. Choice of blocking technique might be guided by samples and the antibodies used. The commonest everlasting blocking brokers embody bovine serum albumin (BSA), non-fats milk, regular goat serum, casein and fish gelatin (Table 1.).
Table 1. Proteins used as blocking brokers in Western blotting
| Protein Recommended | focus | Buffers Membrane | compatibility |
|---|---|---|---|
| BSA | 0.2-5% (W/V) | Tris-buffered saline (TBS)/phosphate buffered saline (PBS) | Nitrocellulose Polyvinylidine difluoride (PVDF) |
| Non-fat milk | 3-5% (W/V) | TBS, PBS | Nitrocellulose PVDF |
| Amersham ECL Prime Blocking Agent | 2-5% (W/V) | TBS, PBS | Nitrocellulose PVDF |
| Casein | 1% (W/V) | TBS | Nitrocellulose PVDF |
| Fish gelatin | 2-10% (W/V) | TBS, PBS | Nitrocellulose PVDF |
| Serum | 1-5% (V/V) | TBS, PBS | Nitrocellulose PVDF |
As every antibody-antigen pair has distinctive traits, no single blocking agent is right for each Western blotting course of. Determining the very best blocking agent and optimum focus are key steps for the success of immune detection. PBS or TBS are generally used as buffers for blocking brokers. Common blocking buffers together with 5% non-fats dry milk or BSA in a TBS-tween answer. However, don’t use dry milk answer when probing with phosphor-particular antibodies, as it will probably trigger excessive background from its endogenous phosphoprotein, casein. It is necessary to soak the blotted membrane in freshly ready blocking agent for 30 min to 2 h at room temperature with fixed agitation. Alternatively, soaking the membrane for 1 h a 37°C or in a single day at 4°C may also help remedy some persistent background points. Decant the block answer and wash with TBS-tween for five minutes.
2. Primary antibody incubation
Following the blocking step, the protein of curiosity will be detected utilizing antibodies. This following course of are similar to ELISA. Many ideas and cautions in ELISA are equally utilized in western blot. Both monoclonal and polyclonal antibodies can be utilized for Western blotting evaluation (Table 2). The most two necessary normal whereas selecting an antibody are: 1) climate it will probably acknowledge the denatured proteins; and a pair of) climate it will probably trigger cross response. Polyclonal antibodies are typically extra delicate, however are much less particular than monoclonal antibodies. Monoclonal antibodies, alternatively, are typically extra particular however much less delicate. Polyclonal antibodies are normally chosen for his or her comparatively cheaper price and fewer time consuming to provide.
• Primary antibodies needs to be raised in species as distinct as doable from the pattern species: it’s higher to lift a major antibody in opposition to a mouse protein in a rabbit, for instance, moderately than a rat.
Table 2. Difference between poly and mono clonal antibodies.
| Signal | specificity | Advantages | Disadvantages | |
|---|---|---|---|---|
| polyclonal antibody | good | good, however have some background | Most can acknowledge denatured protein | Not straightforward to repeat, someday with excessive background |
| monoclonal antibody | differ between antibodies | Best, however might have crossreaction | Fine specificity, not restrict by useful resource | Most can not acknowledge denatured protein |
| combined monoclonal antibody | Best | Best | Strong sign, superb specificity, not restrict by resouce | Easy to acquire |
Dilute the first antibody in a blocking buffer on the focus really useful to the datasheet. Incubate in a single day at Four levels Celsius with light shaking. A really useful choice step is to additionally use a optimistic loading management antibody which permits the consumer to confirm equal quantities of whole protein had been loaded into every properly and aides in troubleshooting by eradicating any uncertainties with the western blot process. The subsequent day, decant off the first antibody answer and wash the membrane with massive volumes of TBS-tween and vigorous agitation 5 instances for five minutes every. These stringent washes are extraordinarily necessary for eradicating non-particular background indicators.
3. Secondary antibody incubation
All kinds of secondary antibodies are commercially obtainable. The alternative of secondary antibodies rely firstly on the species by which the first antibody was produced. For instance, the first antibody was of the IgG isotype and produced in goat, the secondary antibody have to be an anti-goat IgG antibody produced in one other species as it’s going to bind to the Fc area of the first antibody. Although there isn’t a strict rule, secondary antibodies raised in sure host species might result in excessive background ranges.
The procedures for incubation of the secondary antibody answer and the membrane are primarily much like these described for the first antibody. Dilute the secondary antibody in blocking buffer and incubate the membrane for 1h in room temperature on the focus really useful on the information sheet. Decant secondary antibody and wash the membrane with massive quantity of TBS-Tween and vigorous agitation 5 instances for five minutes every, and able to the subsequent detection part.
See Our Secondary Antibody Products
4. Coloration/Visualization
A wide range of detection techniques, primarily based on chemiluminescence, chemifluorescence, fluorescence, chromogenic or radioisotopic detection can be found. The coloration/visualization system are additionally very related with ELISA. See Immunoassay and Chemiluminescence Immunoassay Guide for additional perceive the detection system.
The commonest, most delicate and most cheap detection methodology is the electrochemiluminescence (ECL) system. This methodology make the most of the HRP enzyme, which was conjugated to the secondary antibody to catalyze the ECL response and produce gentle. The gentle is then gathered by detection machine and print onto x-ray movie and developed or digitized with assistance from specialised CCD digicam delicate sufficient for detection. There are two sorts of ECL reagents: reagent A and reagent B, we combined these two ECL reagents in 1:1 ratio, and incubate the membrane into the reagent for 3-5 minutes with out agitation. After incubation, decant the ECL combination and use a wipe to wipe off extra answer from the nook of the membrane. Place the membrane in a transparent plastic wrap corresponding to a sheet protector to stop drying. Both movie and digicam system enable to manually regulate the publicity time with a purpose to guarantee an image excellent western blot. Relative band density will be quantified with commercially obtainable software program. Proper molecular weight can be verified by evaluating band measurement to the molecular weight ladder.
Appendix:
Table 3. Commonly used Western Blot Reagents Recipe
30% Polyacrylamide | Acrylamide monomer: 29g; Methylene Diacrylamide: 1g; Dilute with ddH2O to 100ml quantity at 37°C |
| 1.5M Tris-HCl (PH8.8) | Tris: 90.85g Dissolve in 400 ml ddH2 and add to ultimate quantity of 500ml. Use concentrated HCl to regulate pH to eight.8 |
| 1.0M Tris-HCl (PH6.8) | Tris: 60.5g Dissolve in 400 ml ddH2 and add to ultimate quantity of 500ml. Use concentrated HCl to regulate pH to six.8 |
| 0.5M Tris-HCl (PH6.8) | Tris: 30g Dissolve in 400 ml ddH2 and add to ultimate quantity of 500ml. Use concentrated HCl to regulate pH to six.8 |
| 10% SDS (PH7.2) | SDS: 10.Zero g, dissolve in 80ml ddH2O, and add to ultimate quantity of 100ml. maintain 68°C to assist dissolve. Use concentrated HCl to regulate pH to 7.2 |
| 10% APS (ammonium persulfate) | AP: 1.0g, dissolve in ddH2O and add to quantity of 10ml. Attention: AP can simply maintain in 2 weeks at 4°C, higher use 0.2ml aliquots at -20°C for storage. |
| 10×Electrophoresis Buffer | Tris: 30.Three g; Glycine: 144 g; SDS: 10 g; Dissolve in 800ml ddH2O and add to quantity of 1L. |
| 10×Transfer buffer (with out methanol) | Tris: 30.Three g; Glycine: 144.1 g; Dissolve in 900ml ddH2O and add to quantity of 1000ml. (Add lower than 0.5% SDS for big protein) |
| 1×Transfer buffer | 1L (1×Transfer buffer)=100ml (10×Transfer buffer) + 700ml (ddH2O) + 200ml Methanol (Add earlier than use) |
| 10×TBS | Tris: 24.23 g; NaCl: 80.06 g; Dissolve in 800ml ddH2O and add to quantity of 1L. Use concentrated HCl to regulate pH to 7.6 |
| 1×TBS-Tween | 100ml 10×TBS, add 5ml 20% Tween 20 or 1ml Tween 20, Add ddH2O to quantity of 1L (Attention: Add Tween 20 slowly alongside the beaker wall, or it’s going to deliver out bubbles.) |
| 5% Blocking buffer (non-fats milk) | Non-fat milk: 5g Dissolve in 100ml TBST and properly combined. |
| Coomassie Brillant Blue answer (1L) | Coomassie R-250: 1.0g Methanol: 500ml Glacial acetic acid: 100ml ddH2O: 400ml Could be retailer for six months at room temperature. Use filter paper to make filtration if precipitation. |
| 10× Ponceau Red | Ponceau purple: 2g Trichloroacetic acid: 30g Sulfosalicylic acid: 30g Dissolve in 100ml ddH2O |
| Destaining answer | Methanol: 50ml Glacial acetic acid: 70ml Dissolve in 880ml dd H2O. retailer for 1 month at room temperature |
| 2×SDS loading buffer (10mL) | 0.5M Tris-HCl: 2mL; 10% SDS: 4mL; Glycerin: 2mL; β-Mercaptoethanol: 140μl; Bromophenol blue: 0.1mg |
| 20% Tween 20 Stock answer | Add 20mL Tween 20 into 100mL 1×TBS |
• Western Blot Trouble Shooting
For the difficulty capturing of frequent causes of surprising or sudden bands, no bands, faint bands or weak sign, excessive background on the blot and extra, please learn our WB TROUBLESHOOTING TIPS.
References:
| 1. | Kurien B T, Scofield R H. Western Blotting: An Introduction[J]. Western Blotting: Methods and Protocols, 2015: 17-30. |
| 2. | Hnasko T S, Hnasko R M. The western blot[J]. ELISA: Methods and Protocols, 2015: 87-96. |
| 3. | Manoussopoulos I N, Tsagris M. Native electrophoresis and western blot evaluation: methodology and functions[J]. Protein Blotting and Detection: Methods and Protocols, 2009: 277-287. |
| 4. | Hirano S. Western blot evaluation[J]. Nanotoxicity: Methods and Protocols, 2012: 87-97. |
| 5. | Hughes A J, Spelke D P, Xu Z, et al. Single-cell western blotting[J]. Nature strategies, 2014, 11(7): 749-755. |
| 6. | Kang C C, Yamauchi Ok A, Vlassakis J, et al. Single cell-decision western blotting[J]. Nature protocols, 2016, 11(8): 1508-1530. |
| 7. | Jensen E C. The fundamentals of western blotting[J]. The anatomical report, 2012, 295(3): 369-371. |
| 8. | Mahmood T, Yang P C. Western blot: approach, concept, and hassle capturing[J]. North American journal of medical sciences, 2012, 4(9): 429. |